The Future of Post-Market Surveillance in IVD and MD Industry: Leveraging eIFU
In today’s rapidly evolving landscape of healthcare technology, the need for efficient post-market surveillance (PMS) in the In Vitro Diagnostic (IVD) and Medical Device (MD) industry has become more critical than ever. With advancements in digitalization and connectivity, the traditional methods of monitoring the safety and performance of medical devices are being reshaped by innovative solutions. One such solution that holds immense promise is implementation of Electronic Instructions for Use (eIFU).
The Significance of Post-Market Surveillance
Post-market surveillance plays a pivotal role in ensuring and improving the safety and effectiveness of IVDs and MDs once they are commercialized and in use by healthcare providers and patients. It involves the systematic collection, analysis, and interpretation of data related to the real-world performance of medical devices. By monitoring adverse events, device malfunctions, and other safety concerns, regulatory authorities, manufacturers, and healthcare professionals can identify and address potential risks promptly.
Instructions for Use (IFU) have traditionally been provided in printed form accompanying IVD products and medical devices. However, this approach has its limitations, such as the potential for loss or damage of the physical document and difficulty in updating information. Moreover, printed IFUs cannot adapt to the dynamic nature of medical devices or incorporate multimedia elements for enhanced clarity.
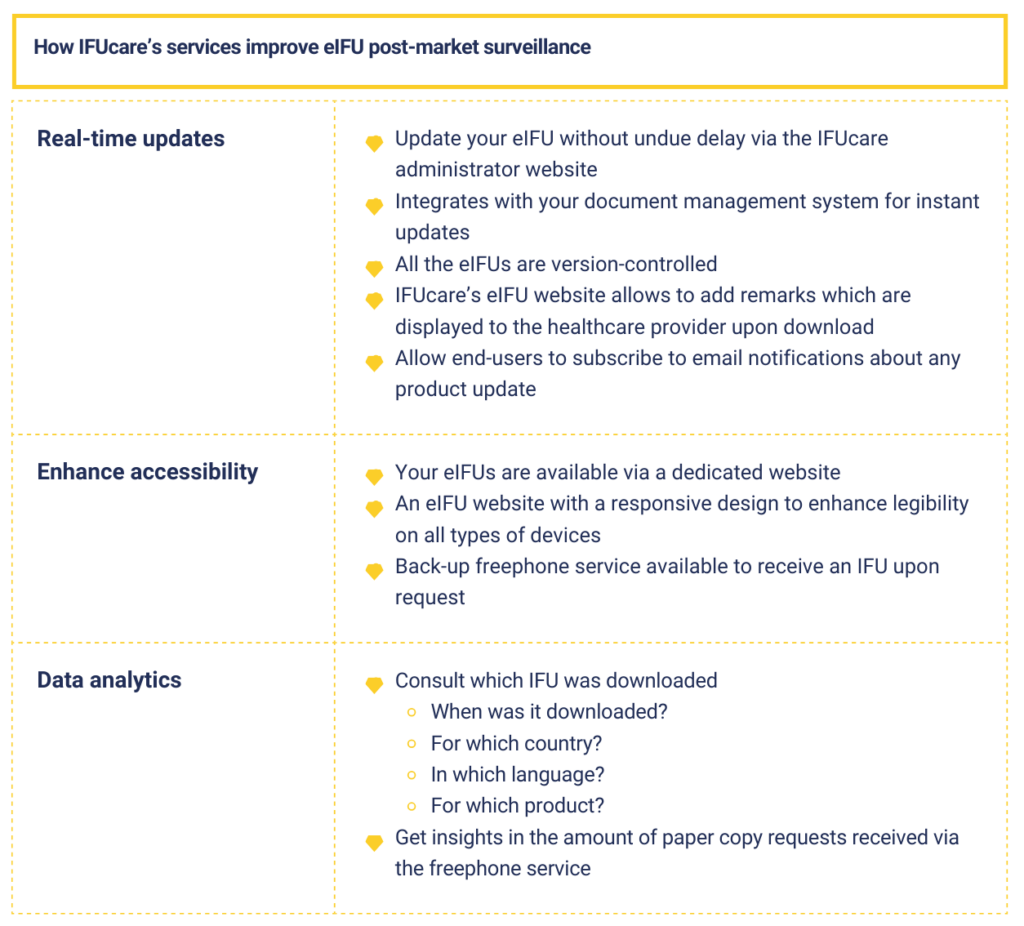
- Real-Time Updates: Unlike printed IFUs, eIFUs can be updated in real-time to reflect the latest information, including safety alerts, device modifications, and regulatory changes. This ensures that healthcare providers always have access to the most current instructions and warnings, reducing the risk of errors and adverse events. Additionally, they can opt to receive notifications about such eIFU updates via email, further ensuring that they stay informed about the latest information. This proactive approach enhances the accessibility of critical updates, empowering healthcare professionals to make informed decisions and prioritize patient safety.
- Enhanced Accessibility: eIFUs can be accessed digitally via various platforms, including mobile devices, tablets, and computers. This accessibility ensures that healthcare professionals can quickly retrieve the information they need, even in challenging clinical environments.
- Data Analytics: eIFUs can be integrated with data analytics platforms to track user interactions and usage patterns. By analyzing how healthcare professionals engage with eIFUs, manufacturers can gain valuable insights into device utilization, target audiences and whether these target markets are reached as expected. This data-driven approach empowers manufacturers to proactively address emerging issues and optimize their processes.
Conclusion
In conclusion, Electronic Instructions for Use (eIFU) represent a shift in post-market surveillance within the In Vitro Diagnostic (IVD) and Medical Device (MD) industry. By leveraging digitalization, interactivity, and real-time updates, eIFUs have the potential revolutionize post-market surveillance and contribute to safer, more effective patient care.
Software requirements for a compliant eIFU website development
CONTACT US
ISO 13485 and ISO 27001 certified
Pas 257, 2440 Geel BELGIUM
+32 (0)14 49 04 22
FAQ
Privacy Policy | Terms & Conditions | Cookie Policy
IFUcare is a brand name of Qarad
IFUcare and Qarad are part of the

