The added value of IFUcare's multilingual freephone service to their eIFU solution
Clients can rely on IFUcare’s eIFU (electronic Instructions for Use) solution to gain immediate access to their documents at any time. On top of the eIFU website, where customers can download the necessary documents themselves, they also provide a free telephone service so clients can request a paper copy at any time of the day or night, and in any language.
Government regulations for medical devices (MDs[1]) and in vitro diagnostic products (IVD[2]) allow manufacturers to provide IFUs digitally in the form of portable electronic storage media (e.g. CD or USB) or via the company’s website.
Mandatory free copies upon request
Although these digital IFUs are allowed, MD and IVD manufacturers are still obliged to provide an alternative means so clients can obtain paper copies on request. IFUcare’s free telephone service guarantees quick and efficient handling of these requests without any burden to the manufacturer.
In IFUcare’s experience, manufacturers prefer a website over CD or USB as it is the most accessible option. However, simply allowing digital IFUs to be downloaded from a website is not sufficient. One of the most challenging requirements for a compliant eIFU solution is the obligation on a manufacturer to be able to provide their customers with paper copies of IFUs on request, free of charge and within a specific timeframe.
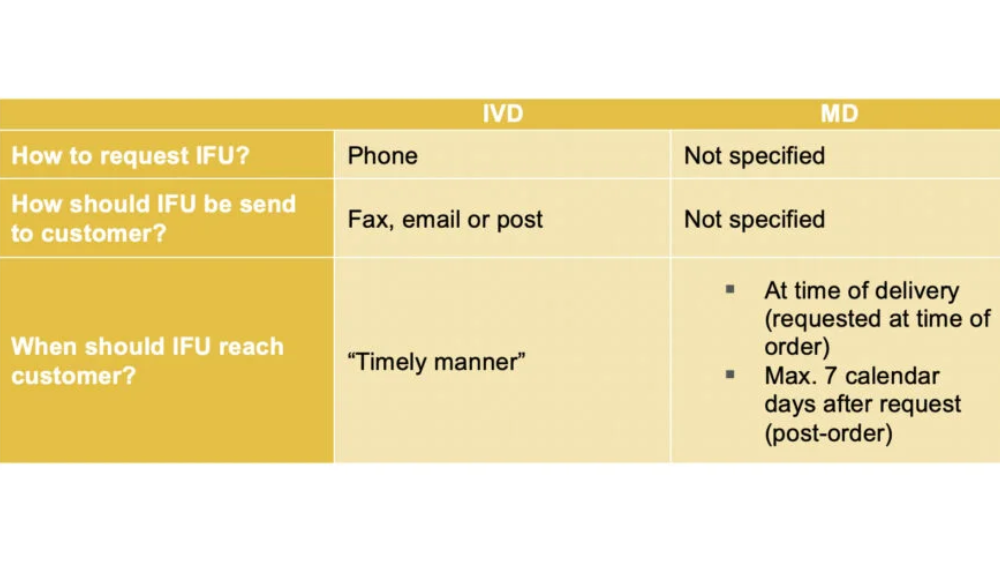
Although there are some differences between the requirements for either Medical device or IVD manufacturers to fulfil such requests, it is equally challenging.
The MEDDEV rules for IVDs state that a free telephone contact number must be provided so that customers can ask for copies of IFUs over the phone. These should then be sent to them via fax, by email or post in ‘a timely manner’.
The MD regulation is more flexible on how that free copy can be requested. It only mentions that a system should be in place, but it does not specify a preferred method for how clients can request paper copies.
However, the regulation for MD is more restrictive when it comes to a deadline when the paper copy should reach the customer. It clearly states that IFUs must be provided either with the order at the time of delivery, or within seven calendar days if the customer asks for it post-order.
Challenges when providing printed IFUs after delivery of goods
Although receiving and handling these requests sounds simple, it is definitely not easy. Consider a situation where customers must be able to request an IFU over the phone. Such a request could be made 24/7/365, from anywhere in the world, in a variety of languages. An international call centre solution might be considered, but this is usually costly.
Another concern is the actual printing of the requested IFUs and their onward delivery to the customer, either within seven calendar days or a ‘reasonable time’.
This might raise the following concerns:
- Will you organize in-house printing, or do you outsource the task?
- How to make sure the IFU gets to the customer in the right timeframe?
- Which department will manage the process?
- Has provision of this after sales service been costed to the goods sold?
- What if the system or process responsible for this service breaks down?
On top of all this, you have to find a way through this organizational nightmare whilst working in a strict quality-controlled environment. How will you prove to the authorities that you handled the paper copy requests according to governing regulations, and that the documents arrived at the customer’s site in the allotted time? Especially when you also have to take privacy rules into consideration.
By outsourcing your eIFU project to IFUcare, all these concerns are redundant. IFUcare developed its eIFU solution many years ago and its people are some of the most experienced professionals, who have been working in the industry for decades.
IFUcare’s eIFU solution combines a compliant website with a freephone service that can provide paper copies to clients quickly and efficiently.
Software requirements for a compliant eIFU website development
[1] COMMISSION REGULATION (EU) No 207/2012 of 9 March 2012 on Electronic Instructions for Use (eIFU) for use of medical devices
COMMISSION IMPLEMENTING REGULATION (EU) 2021/2226 of 14 December 2021 laying down rules for the application of Regulation 2017/745 of the European Parliament and of the Council as regards electronic instructions for use of medical devices
[2] MEDDEV 2.14/3 rev 1 Supply of Instructions for Use (IFU) and other information for In-vitro Diagnostic (IVD) Medical Devices
CONTACT US
ISO 13485 and ISO 27001 certified
Pas 257, 2440 Geel BELGIUM
+32 (0)14 49 04 22
FAQ
Privacy Policy | Terms & Conditions | Cookie Policy
IFUcare is a brand name of Qarad
IFUcare and Qarad are part of the

